Pharmacoepidemiology
Reconciling conflicting results from trials of 17P and preterm birth Arti Virkud* Arti Virkud Eric Tchetgen Tchetgen Enrique F. Schisterman Beth Pineles Lisa Levine Stephen R. Cole Stefanie N. Hinkle Sunni Mumford Sean Blackwell Alan Peaceman Ellen Caniglia
The Meis et al. trial identified a clinically significant benefit of 17-alpha-hydroxyprogesterone caproate (17P) on reducing the risk of recurrent spontaneous preterm birth (SPTB). However, the confirmatory PROLONG trial identified no effect of 17P on SPTB. PROLONG had a different study population than Meis, despite efforts to recruit similar trial participants. Meis participants had a higher count of prior SPTBs, higher BMI, and were more likely to smoke during pregnancy. Approximately 59% of Meis participants identified as non-Hispanic Black, compared to fewer than 7% of PROLONG. We implemented state of the art machine learning techniques for transportability to investigate whether the conflicting results might be driven by measured differences.
We first estimated RDs for SPTB by treatment arm in both trials, adjusting for measured baseline variables. We then transported the risk of SPTB in each arm of Meis to estimate the causal RD in the PROLONG population and vice versa from PROLONG to the Meis population, using direct standardization via outcome regression, inverse odds weighting, and doubly robust estimators leveraging ensemble learning via SuperLearner and cross fitting. We additionally evaluated if the results changed among individuals enrolled in the US and those with only one prior SPTB. We obtained 95% CIs via nonparametric bootstrap.
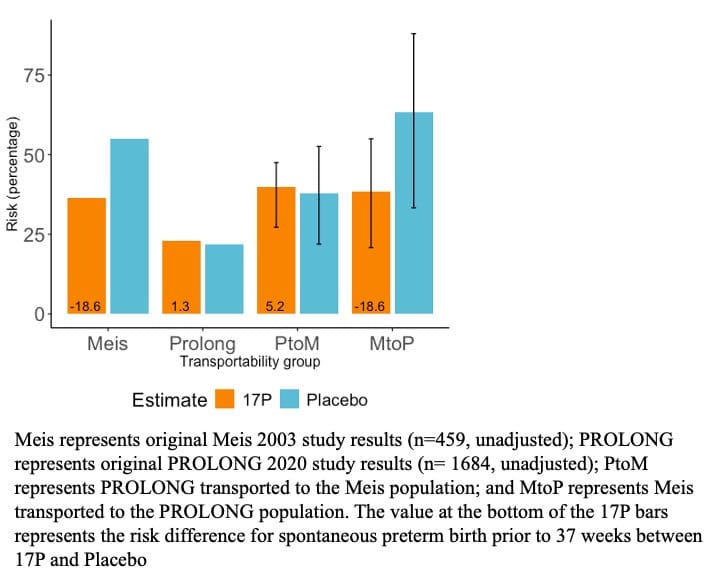
Comparing 17P to placebo, the adjusted RD (95% CIs) was -16.4% (-26.7%, -6.9%) in Meis and 1.1% (-3.3%, 5.2%) in PROLONG. Comparing 17P to placebo, the doubly robust RD was -18.6% (-57.3%, 6.2%) when transporting Meis to PROLONG and 5.2% (-18.0%, 17.4%) when transporting PROLONG to Meis (Fig 1). These results were consistent across the other estimators and sensitivity analyses.
Existing methods for transportability approach did not reconcile the conflicting results between the two trials of 17P and SPTB. There may be several explanations for these results including unmeasured effect measure modification between the trials.