Study Design
Bayesian methods to evaluate azithromycin mass drug administration for child mortality reduction in Niger Huiyu Hu* Huiyu Hu Ahmed M. Arzika Kieran S. O’Brien Elodie Lebas Ramatou Maliki Travis C. Porco Benjamin F. Arnold Thomas M. Lietman
Background
The MORDOR trial showed an 18% reduction in all-cause mortality among children aged 1–59 months through biannual azithromycin distribution in Niger. Building on these results, the AVENIR trial, a cluster adaptive randomized controlled study, evaluated age-based mass drug administration (MDA) strategies to prevent child mortality. Bayesian methods provide flexibility to incorporate prior knowledge and adapt to evolving data, overcoming limitations of traditional frequentist trials.
Methods
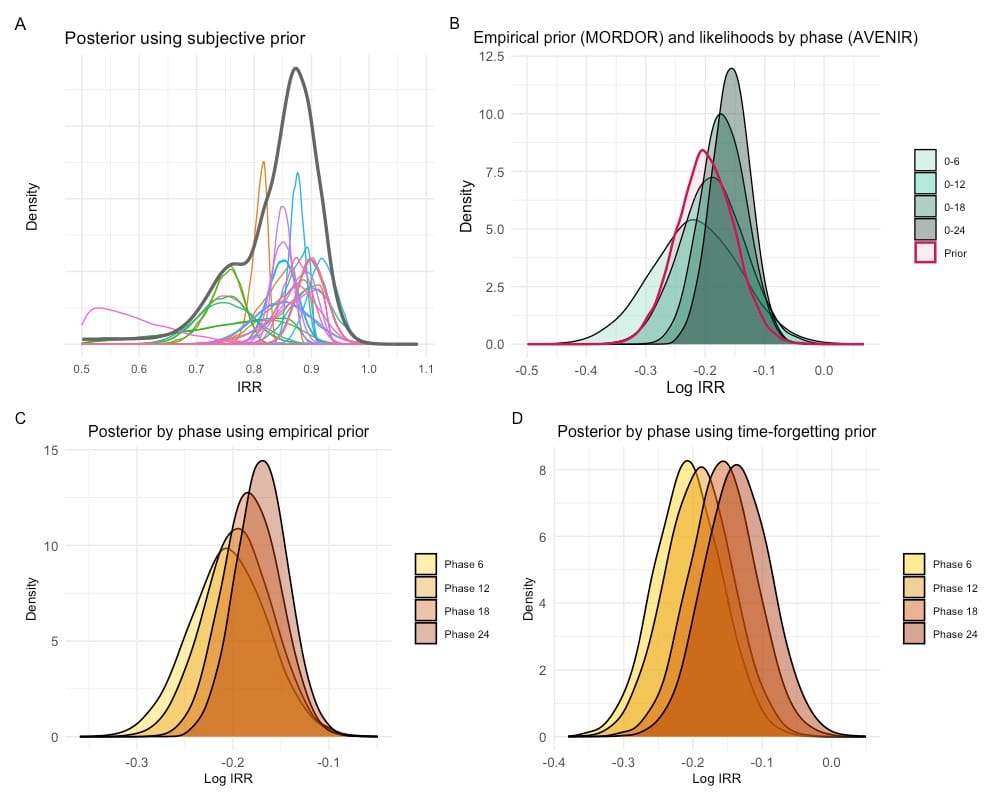
We employed a Bayesian framework with three types of prior distributions—subjective, empirical, and time-forgetting. Subjective priors were collected from 31 public health experts at a topic-specific meeting, fitting a skew-normal distribution to individual’s beliefs. Empirical priors were derived from the previous MORDOR trial. With MORDOR data, time-forgetting priors reduced reliance on early data by down-weighting old information as new evidence accumulated, maintaining consistent variance and keeping posterior information constant over time. Mortality data from AVENIR phases (0–6, 0–12, 0–18, and 0–24 months) were sequentially incorporated via Bayes’ theorem to estimate posterior distributions of azithromycin’s effect.
Results
Posterior estimates confirmed meaningful reductions in child mortality. Subjective priors estimated a 13.6% reduction (95% credible interval [CrI]: 7.7%–19.5%) (Figure A). Using empirical priors from MORDOR (Figure B), posterior estimates showed increasingly precise estimates, with the posterior means decreasing from an 18.1% reduction (95% CrI: 10.0%–25.5%) at 0–6 months to 15.7% (95% CrI: 9.6%–21.5%) at 0–24 months (Figure C). Time-forgetting priors enabled dynamic updates, with posterior means evolving from an estimated 18.7% reduction in mortality (95% CrI: 12.3%–24.8%) at 0–6 months to 12.8% reduction (95% CrI: 8.1%–17.4%) at 18–24 months (Figure D).
Conclusions
Bayesian methods integrating expert beliefs, empirical data, and time-forgetting mechanism provide a framework for evaluating azithromycin MDA efficacy over time. This approach enables real-time updates for on-going trials, supports data-driven decisions, and informs programmatic implementation to reduce childhood mortality in low-resource settings.