Substance Use
Reducing prescription opioid dose and duration to reduce risk of opioid use disorder among patients with musculoskeletal pain Shodai Inose* Shodai Inose Nicholas T. Williams Katherine L. Hoffman Allison Perry Iván Díaz Kara E. Rudolph
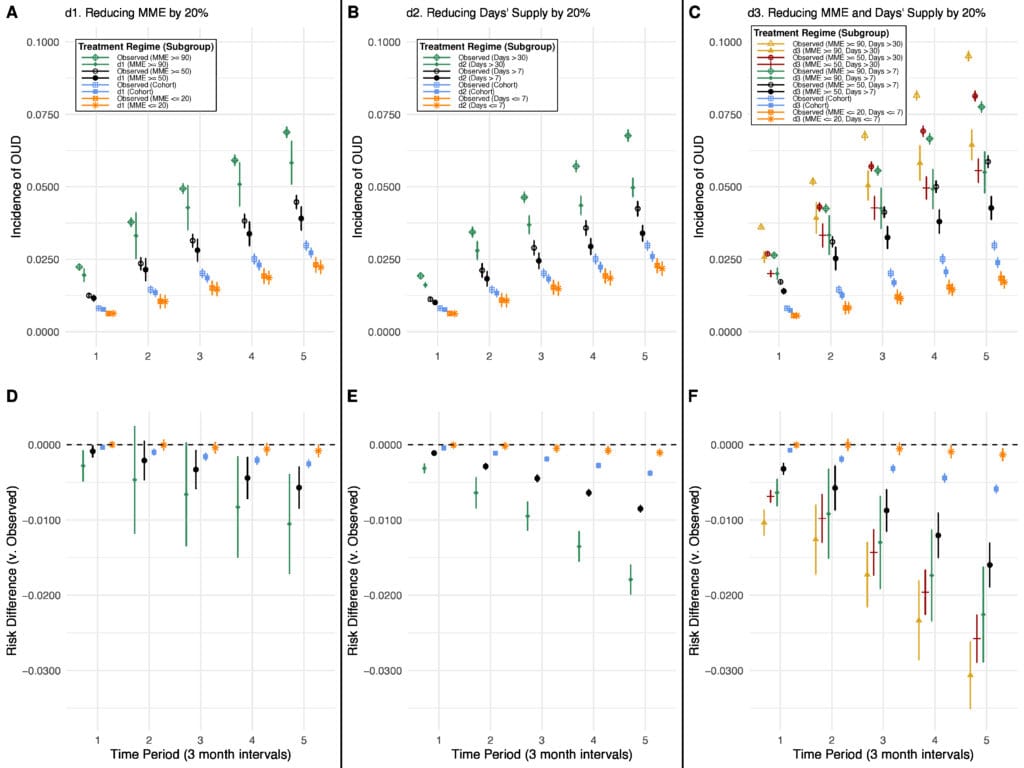
Although the U.S. Centers for Disease Control and Prevention’s (CDC) opioid prescribing guidelines (2022) recommend initiation of opioids at the lowest effective dose for the shortest required time, they do not discuss the joint effects of these prescribing characteristics and are written to apply broadly to all patients. Due to the prevalence of high-risk levels of opioid prescribing for musculoskeletal (MSK) pain, we examined the effects of applying modest opioid dose and/or duration reduction policies to adult Medicaid patients with newly diagnosed MSK pain who were prescribed opioids (n=229,434) on the risk of developing opioid use disorder (OUD) over 15 months. We divided our cohort into subgroups by prescription “riskiness” based on maximum daily morphine milligram equivalents (MME) and the number of days supplied for all opioid prescriptions in the 3 months following diagnosis, using prescribing cutoffs based on CDC guidelines and state laws limiting opioid prescription. We used a novel approach to estimate the effects of localized modified treatment policies within subgroups (a generalization of the average treatment effect on the treated). Reducing both opioid prescription dose and duration by 20% across the cohort resulted in a statistically significant, but clinically modest, decreased risk of OUD (absolute RD: -0.59%). In contrast, larger, clinically significant reductions in risk >1 percentage point were observed when assessing effects of 20% reductions among the following risky subgroups independently: 1) reducing dose for those with ≥90 MME (RD: -1.05%), 2) reducing days supplied for prescriptions >30 days (RD: -1.79%), and 3) reducing both dose and duration for those with ≥50 MME for >7 days (RD: -1.60%; -3.06% among those with ≥90 MME for >30 days). These findings suggest that reductions in opioid prescribing may have limited impact on OUD risk when applied broadly, but may meaningfully reduce risk when applied to those with riskier prescriptions.