Substance Use
Comparative effectiveness of extended release naltrexone and sublingual buprenorphine for treatment of opioid use disorder among Medicaid beneficiaries Rachael Ross* Rachael Ross Edward Nunes Mark Olfson Matisyahu Shulman Noa Krawczyk Elizabeth Stuart Kara Rudolph
Introduction: Extended release naltrexone (XR-NTX) and sublingual buprenorphine (SL-BUP) are medications for treatment of opioid use disorder. In contrast to methadone, XR-NTX and SL-BUP can be prescribed in any healthcare setting. There is limited real-world evidence to inform the choice between XR-NTX and SL-BUP. We compared their treatment effectiveness in a real-world population.
Methods: We created an active comparator, new user cohort using 2016-2019 Medicaid claims data from CA and NJ. We included adults who initiated XR-NTX or SL-BUP for OUD maintenance treatment and did not use any medication for OUD in the prior 90-days. We estimated comparative treatment effects of the medications on medication discontinuation and overdose, over 180 days (6 months) following initiation. We used a sequentially doubly robust estimator with nuisance models adjusted for baseline confounders estimated flexibly using SuperLearner.
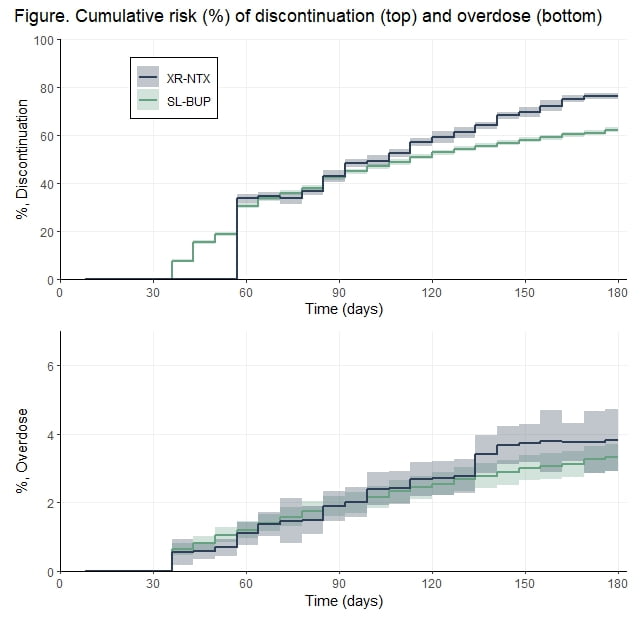
Results: Our cohort included 1,755 XR-NTX and 9,886 SL-BUP patients. The figure presents the adjusted cumulative risk (as %) for medication discontinuation (top) and overdose (bottom). Patients were immune to discontinuation until day 60 after initiation of XR-NTX (injection duration + 31-day grace period) so initially SL-BUP discontinuation was higher, but by 180 days XR-NTX initiating patients were more likely than SL-BUP initiating patients to discontinue: 76% vs. 62%, respectively; RD 14% (95% CI 13, 16). There was minimal difference in the risk of overdose: 3.8% vs. 3.3%; RD 0.5% (95% CI -0.5, 1.5). Our results were robust to several sensitivity analyses.
Conclusions: Longer medication retention is important because risks of negative outcomes are elevated after discontinuation. Our results support selection of SL-BUP over XR-NTX. However, most patients discontinued medication by 6 months indicating that more effective tools are needed to improve treatment retention, particularly after XR-NTX initiation.