Methods/Statistics
Visualizations for non-inferiority trial simulations with applications in quantile regression Hayden Smith* Hayden Smith Geoffrey Wall
Introduction: New pharmacological agents and delivery methods can be compared to standard therapies using non-inferiority trials. Sample size and power determinations for these trials can be calculated using data simulations. Objective: to present a sample size determination with multiple visualizations for non-inferiority trial-based data simulations.
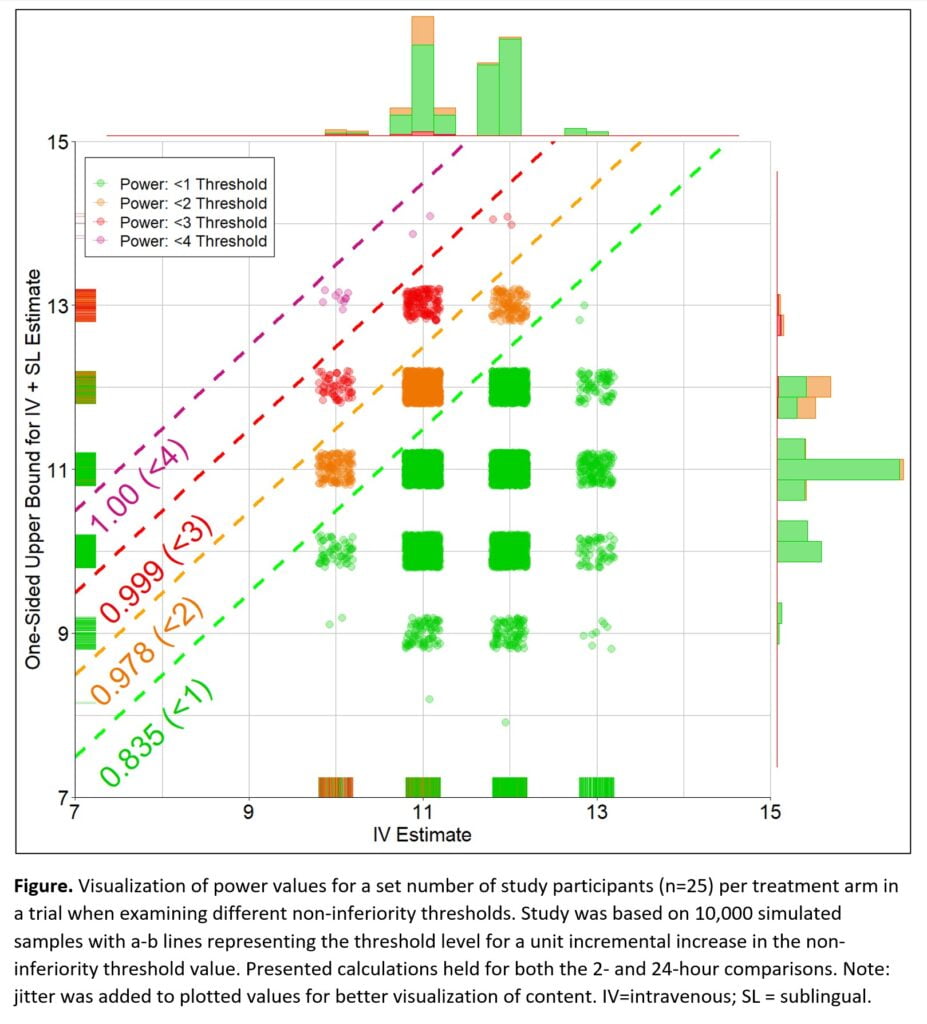
Methods: A trial was designed to compare intravenous dexmedetomidine (IV dex [standard therapy]) to IV dex plus adjunctive sublingual (SL) dex in reducing alcohol withdrawal syndrome symptoms in Intensive Care Unit patients. The estimand was the median (tau=0.5) difference in modified Minnesota Detoxification Scale (mMINDS) scores at 2- and 24-hours after treatment initiation across study arms. Differences were calculated using quantile regression. Sample size and power levels were determined based on 10,000 simulated samples, with an expected IV dex estimate of N~(12, 2) and IV + SL dex contrast estimate of N~(-2, 2). The non-inferiority threshold value was a contrast SL dex score </= 1.
Results: Simulations revealed 25 subjects per treatment arm would result in an alpha of 0.05 and beta of 0.97 for the contrasted median SL dex mMINDS score equal or below the threshold, see Figure. Also presented at the conference will be additional visualizations and statistical code (i.e., SAS and R) for simulations and interim analyses. Lastly, various quantile regression methods (e.g., conditional, Bayesian, heterogenous treatment effects, quantile treatment effects/doubly robust, and tree or neural network-based estimates) are currently available and their strengths and limitations in simulation studies will be discussed.
Conclusions: The presented simulation and visualization methods can be employed in observational and experimental study designs. Of note, the developed figures can help communicate explicit study design details to sponsoring agencies and Institutional Review Boards.