Infectious Disease
Double blind randomized controlled trial to evaluate the safety of Lactobacillus rhamnosus GG ATCC 53103 (LGG) vs. placebo in elderly subjects receiving standard-dose trivalent inactivated influenza vaccine Sam Gebeh* Sam Gebeh Sowmya Rao Patricia Hibberd
Background: Improving the immune response of elderly (≥65 years) to the influenza vaccine is a vital public health goal. We conducted a Phase 1 trial of a probiotic as a potential adjuvant, during the 2011-2012 flu season as required by the Food and Drug Administration. It was previously difficult to publish the safety and preliminary immunogenicity results from our small Phase I study, but given the current interest in the universal flu vaccine, our results may provide preliminary data for future studies of probiotics as potential vaccine immune adjuvants.
Objectives: (i) To assess the safety and tolerability of Lactobacillus rhamnosus GG (LGG) vs. placebo taken orally by elderly subjects for 28 days after standard-dose trivalent inactivated influenza vaccine (TIV); (ii) To measure anti-influenza systemic immune response (hemagglutinin inhibition (HAI) titers) from baseline to the end of the flu season.
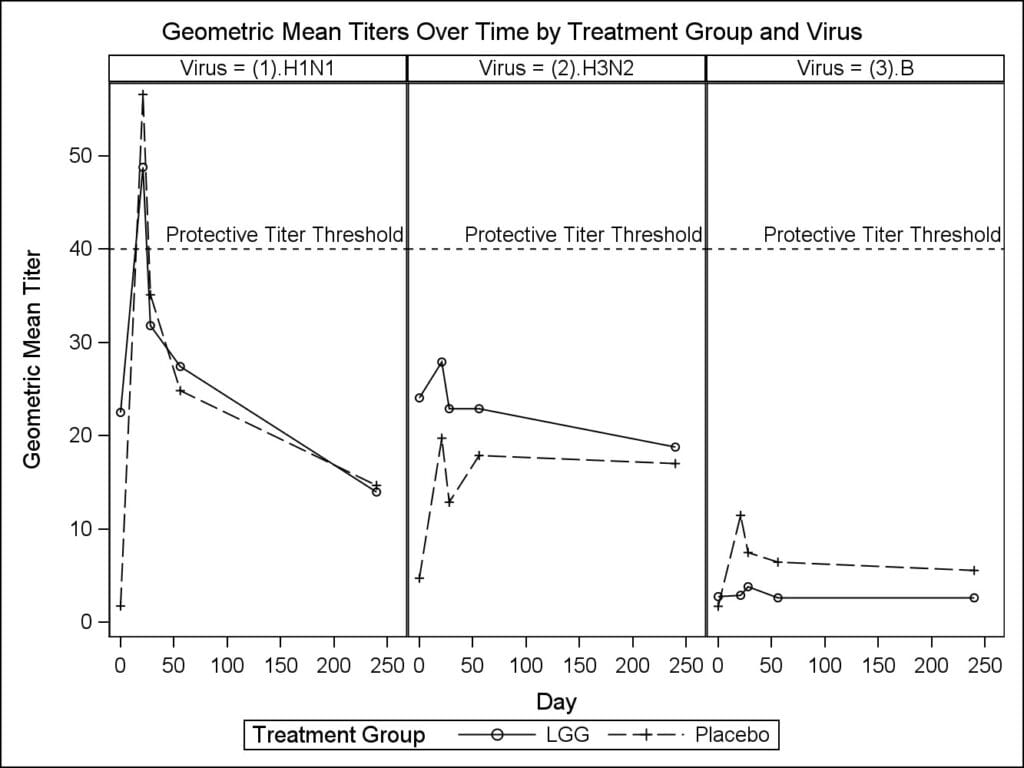
Methods: Our double blinded randomized Phase I trial enrolled 28 elderly subjects who received TIV and were randomized to 1×1010 CFU LGG or placebo twice daily for 28 days. We assessed subjects for adverse events throughout the study and measured HAI titers (H1N1, H3N2, B) at baseline, day 21, 28, 56, and at the end of the flu season. We got summary statistics for adverse events. We used a 2-sample t-test to compare the change in log geometric mean titers from baseline to each time point and Fisher’s exact test to compare proportions achieving protective titers (≥40) between groups.
Results: Of all subjects, 57% receiving LGG and 64% receiving placebo reported treatment related non-serious adverse events. We observed protective geometric mean titers only for H1N1. The change in the average log geometric mean H1N1 titers was 2.5 units lower at each time point, and for H2N3 and B, it was lower only at day 21 for LGG than placebo.
Conclusion: LGG was safe and well tolerated among elderly subjects, but didn’t have an impact on HAI titers.
Trial registration: NCT01368029