Methods/Statistics
Tree-based scan statistics to evaluate drug safety in pediatric populations: sulfamethoxazole/trimethoprim as a positive test case Kelly Fung* Kelly Fung Loreen Straub Timothy Savage Massimiliano Russo Helen Mogun Thomas Deramus Georg Hahn Shirley V. Wang Krista F. Huybrechts
Clinicians treating pediatric patients rely on evidence generated in adults. However, the safety profile of medications may differ for children. Tree-based scan statistic (TBSS) approaches can be used to identify safety issues by screening thousands of potential adverse outcomes while controlling type 1 error, but their performance in pediatric populations has not been evaluated.
Th study objective was to assess whether TBSS can identify rare, serious adverse effects and describe the pattern of unsuspected alerts using the known association of sulfonamides and Stevens-Johnson Syndrome (SJS) as a test case.
Using Medicaid (2008-2018) and MarketScan (2008-2021) databases, we compared children who initiated sulfamethoxazole/trimethoprim (SMX-TMP) to those initiating cephalexin and clindamycin, respectively. The outcome tree was based on hierarchical groupings of diagnostic codes in the International Classification of Disease system. Incident outcomes were identified in the 30 days after treatment initiation, using a 90-day washout. We adjusted for confounders using propensity score overlap weights. A p-value threshold of 0.05 was used to define statistical alerts for potential safety signals.
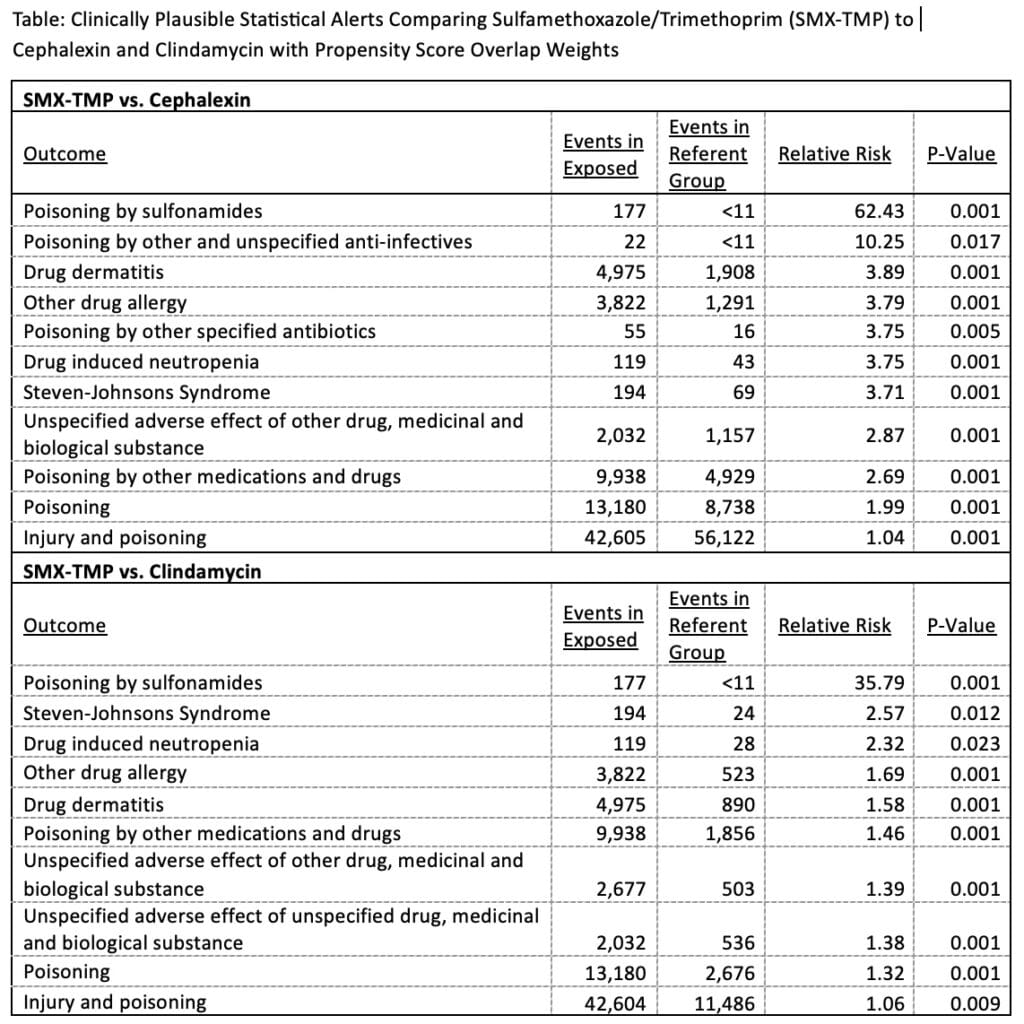
There were 2,939,183 children exposed to SMX-TMP, 3,908,066 to cephalexin, and 755,945 to clindamycin. Comparing SMX-TMP to cephalexin, 278 statistical alerts were detected out of 8,517 outcomes screened. Among these, 11 alerts were considered clinically plausible adverse events; the remainder being related to infections or chronic illnesses. Compared to clindamycin, 144 alerts were identified, 10 of which were clinically plausible. Increased risks for SJS were identified in both comparisons.
TBSS detected the known risk of SJS, a rare but serious condition associated with SMX-TMP, without raising many additional clinically plausible alerts, supporting the feasibility of the approach. Follow-up of these plausible alerts and further evaluation of the TBSS approach will be important.