Pharmacoepidemiology
Study design and comparator choices in evaluating the risk of suicidal ideation and suicidality with semaglutide: A real-world study Qoua Her* Qoua Her Tiansheng Wang Til Sturmer John Buse Virginia Pate Michele Jonsson-Funk Michael Webster-Clark
Semaglutide, a GLP-1 analog, is prescribed for both type 2 diabetes and weight loss. After reports of suicidal ideation (SI) prompted regulatory review, a real-world study estimated a HR of 0.27 for SI comparing new users of semaglutide to prevalent users of weight loss ingredient (WLI) drugs (bupropion, naltrexone, orlistat, phentermine, or topiramate). Robust pharmacoepidemiologic designs with active comparators are warranted.
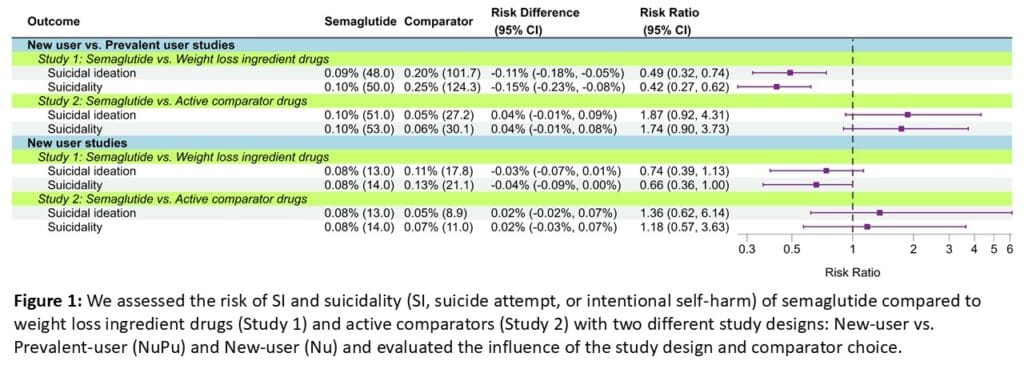
We conducted two different real-world studies in the Merative MarketScan™ Databases. We identified overweight or obese patients who initiated semaglutide, WLI drugs, or active comparators (combination bupropion/naltrexone, orlistat, phentermine, or combination phentermine/topiramate) and followed them for SI and suicidality (SI, suicide attempt, or intentional self-harm). We compared semaglutide to WLI drugs (Study 1) and active comparators (Study 2) using two distinct study designs: New user vs. Prevalent-user (NUPU) and New user (NU) to evaluate the influence of study design and comparator choice. WLI and active comparators patients were weighted to resemble semaglutide initiators to reduce confounding. We estimated risk ratios (RR) for SI and suicidality within the 183 days of drug initiation using Kaplan-Meier methods with 95% confidence intervals from 1,000 bootstrap replicates.
In Study 1, semaglutide was associated with a reduced SI risk (RR 0.49 [0.32–0.73]) versus WLI drugs (NUPU), but this association was attenuated with the NU design (SI: RR 0.74 [0.39–1.13]). In Study 2, semaglutide was associated with a higher SI risk (NUPU: RR 1.87 [1.00–4.22]; NU: RR 1.36 [0.62–6.14]) compared to active comparators. These results were similar when examining suicidality.
Semaglutide was not associated with increased SI or suicidality when using an appropriate study design and compared to active comparators. Careful consideration of study design and appropriate comparator choice are essential in drug safety studies.