Perinatal & Pediatric
Screening for Pediatric Safety Alerts Following Antipsychotic Treatment Initiation using a Tree-Based Scan Statistics Approach Loreen Straub* Loreen Straub Shirley V. Wang Kelly Fung Helen Mogun Massimiliano Russo Georg Hahn Krista F. Huybrechts
Antipsychotics (APs) are commonly prescribed to children. While pediatric studies have provided evidence on adverse events for widely used APs (i.e., aripiprazole, olanzapine, quetiapine, risperidone, ziprasidone), safety information on newer APs (i.e., asenapine, brexpiprazole, cariprazine, lurasidone, paliperidone) remains sparse.
Using tree-based scan statistics (TBSS) to screen a broad range of outcomes, we evaluated whether known associations for common APs could be identified, and whether previously unrecognized adverse effects associated with newer APs would be detected.
We identified children initiating APs (with quetiapine as the reference) within two large US health insurance databases 2000-2021. Leveraging the Multi-Level Clinical Classification tree which groups diagnosis codes into higher-level concepts, we scanned for incident outcomes within six months of AP initiation. Relative risks (RRs) were estimated with propensity score fine-stratification for confounding adjustment. A p-value threshold of <0.1 was used to define statistical alerts.
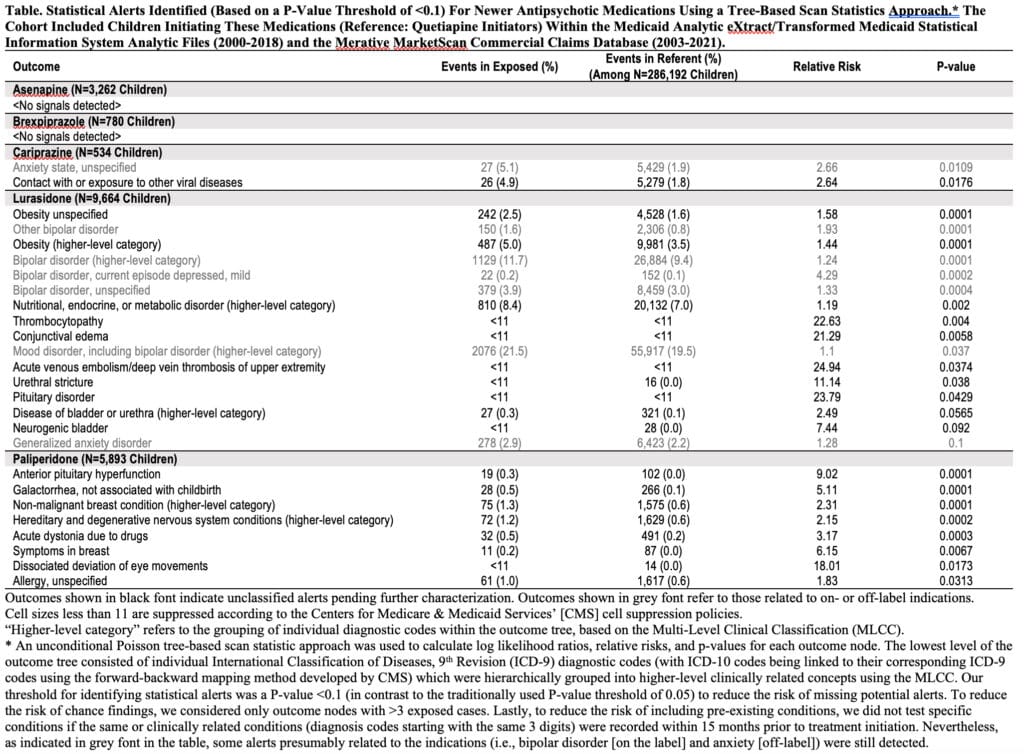
The number of exposed children ranged from 534 (cariprazine) to 664,967 (risperidone). Compared to quetiapine (N=286,192), multiple elevated risks, consistent with previous evidence, were identified for common APs, including endocrine, cardiovascular, hematologic, metabolic, neurologic and immunologic conditions. Among newer APs, no signals were detected for asenapine and brexpiprazole. For cariprazine, one alert for viral disease (RR=2.6) was found. Lurasidone was associated with metabolic, urinary, pituitary and platelet disorders (RR range 1.4-24.9), and paliperidone with extrapyramidal and pituitary disorders and allergies (RR range 1.8-18.0).
The detection of known associations supports TBSS’ feasibility for pediatric drug safety surveillance. Continued monitoring of the newer drugs as more data accrue, in-depth assessment of clinical plausibility and replication in independent data sources remains essential.