Nutrition/Obesity
Harnessing Social Media to Analyze Temporal and Co-occurrence Trends of GLP-1 Agonists Side Effects from 2022 to 2024 Vidur Jain* Vidur Jain Amrutha Alibilli Thu Thi Xuan Nguyen
Background: In recent years, there has been a dramatic influx in the use of glucagon-like peptide 1 (GLP-1 RAs) receptor agonists for weight loss. However, there has been limited research regarding short-term, long-term, and co-occurrent side effects associated with GLP-1 RAs medications.
Objective: This study aims to quantitatively analyze temporal and co-occurrent side effect trends through discussions of GLP-1 RA weight loss medications on Facebook and Instagram from 2022 to 2024, a recent period following the FDA approval of GLP-1 RAs medications, such as Tirzepatide.
Methods: We collected 63,023 posts from January 1, 2022, to May 31, 2024 from Facebook and Instagram posts through CrowdTangle, a public insights tool from Meta. Using English language social media posts from the United States, we examined the effect of side effects in relation to six weight loss medications. All analyses were conducted using Python (version 3) in a Google Colab environment.
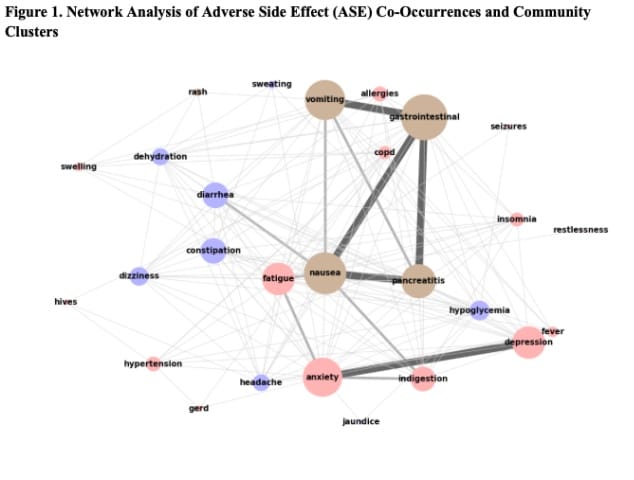
Results: Among posts, 13.8% mentioned side effects. Gastrointestinal (GI) side effects were most common, with 6.5% of Mounjaro posts, 4.3% of GLP-1 RA posts, and 3.6% of Wegovy posts reporting them. Ozempic (Semaglutide) posts linked to depression at 2.49% versus other GLP-1 RAs at 0.16%. Headache (2.00%) and joint pain (1.80%) were the most reported in Tirzepatide and Wegovy posts (0.96%; 0.87%). Node network analysis found GI symptoms (nausea, vomiting, pancreatitis, diarrhea, indigestion) strongly associated together (≥100 mentions), while neurological symptoms (anxiety, depression, insomnia) were moderately correlated together (50-100 mentions).
Conclusion: This social media study highlighted adverse side effects, along with side effect co-occurrence patterns related to GLP-1 RAs medications. These findings underscore the importance of monitoring social media discussions to predict novel, underreported GLP-1 RAs side effects to improve patient care and guide informed public health policy interventions.