COVID-19 Pandemic
Nirmatrelvir-ritonavir (Paxlovid) Initiation Timing and Completion During Acute COVID-19 Ning Zhang* Ning Zhang Jessie K. Edwards Katie R. Mollan Stephen R. Cole Justin Lessler Bonnie E. Shook-Sa William A. Fischer David A. Wohl
Background The oral antiviral Nirmatrelvir-ritonavir (Paxlovid, NMV/r) is effective in treating COVID-19 when initiated within 5 days of symptom onset and continued for 5 days in high-risk individuals, but the real-world timing of NMV/r initiation and discontinuation have not been well described.
Methods Within a large prospective, observational study of people in North Carolina with acute COVID-19, we characterized patterns in NMV/r use among symptomatic individuals who tested positive for SARS-CoV-2 within 7 days of study entry between October 2022 and March 2024. We presented the probability of initiating NMV/r from 0 to 14 days after symptom onset and the probability of remaining on NMV/r at 0 to 6 days after initiation, using inverse probability censoring weighted Kaplan-Meier curves. Analyses were conducted overall and stratified by age, sex at birth, self-reported race, ethnicity, vaccination recency, prior infection(s), residential urbanicity, and risk for progression to severe disease. The average time to NMV/r initiation and discontinuation were reported by individual characteristics.
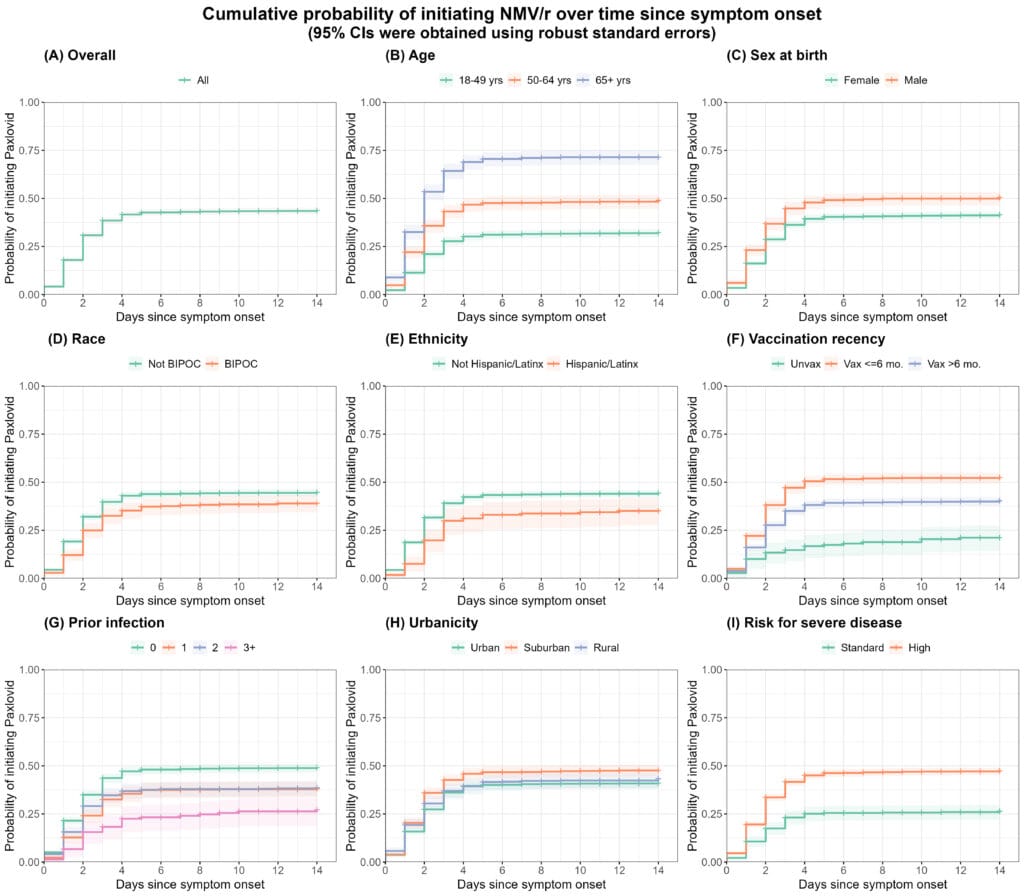
Results Among 3,141 symptomatic participants, the median age was 47 (IQR: 35, 61); 39% received their last SARS-CoV-2 vaccine within the prior 6 months, and 57% were experiencing their first known infection. The estimated probability of initiating NMV/r within 5 days of symptom onset was 0.43 (95% CI: 0.41, 0.44). Participants less likely to initiate NMV/r within 5 days included those who were younger (<65 years of age), female, non-white, Hispanic/Latinx, unvaccinated, or had 3+ prior infections (Figure). Among the 1,243 NMV/r recipients, 7.9% did not complete treatment, and the estimated probability of taking >6 days to finish the full course was 0.10 (95% CI: 0.08, 0.12). The probability of premature discontinuation was similar across groups defined by baseline covariates.
Conclusions Unvaccinated and historically marginalized groups were associated with lower NMV/r use.