Reproductive
Concurrent pregnancy and GLP-1 use is on the rise, but interest in outcomes remain sparse Erin N. Hulland* Erin Hulland Marie-Laure Charpignon Thomas Berkane Maimuna S. Majumder
Glucagon-like peptide-1 receptor agonists (GLP1s) like Ozempic and Wegovy have become household terms nearly overnight, with a recent meteoric rise in internet search popularity in the United States. However, with this rise in interest, there has also been a surge in “off-label” use, described as the acquisition of drugs through means other than via a provider’s prescription or alternate use than that approved by the FDA (e.g. Ozempic for weight loss). This off-label use is often accompanied by a lack of oversight by physicians, sometimes resulting in incorrect dosing, unmonitored sequelae, or other unforeseen effects. One such effect is unintentional pregnancy, as there is increasing evidence of improved fertility among a cohort of GLP-1 users previously facing infertility. Animal trials of Ozempic and Wegovy observed increased rates of miscarriage, fetal anomalies, and fetal demise with GLP1 use during pregnancy; current guidance thus recommends waiting two months after discontinuing GLP1s to get pregnant. Given the recency of the approval of GLP1 use for weight loss, data on such use during pregnancy are sparse, resulting in a dearth of information about pregnancy outcomes; formal cohort studies are ongoing.
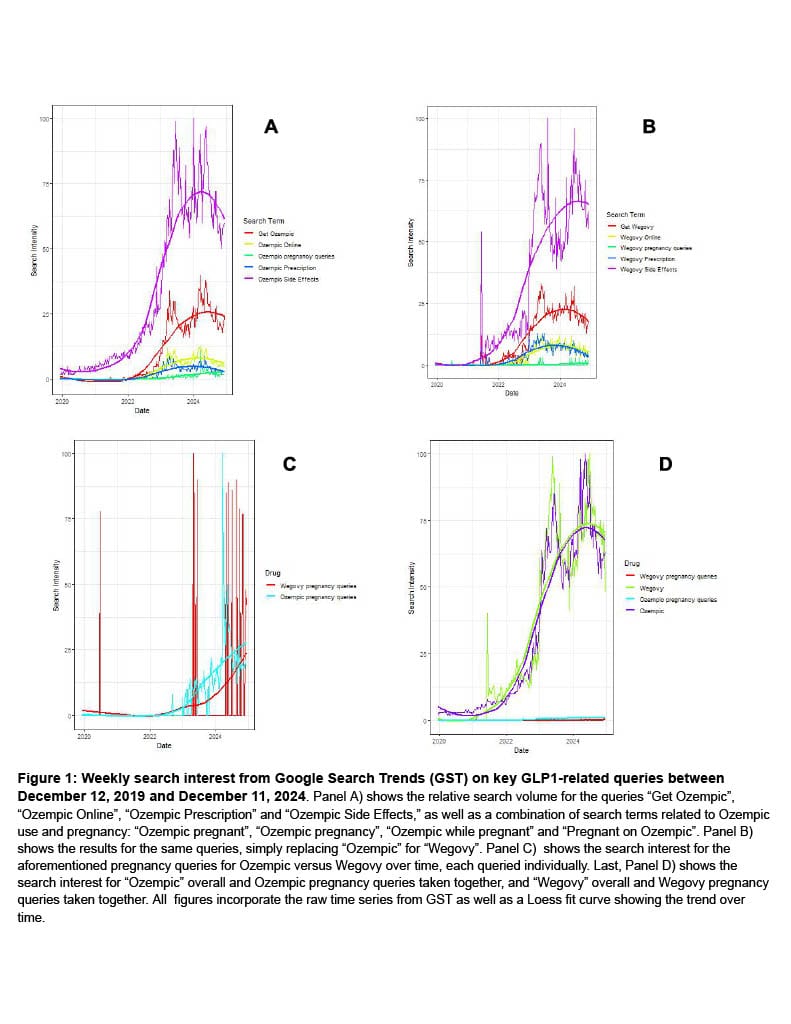
Our analysis of Google Search Trends data for GLP1-related terms revealed markedly lower search interest in pregnancy-related GLP1 compared to other common GLP1-related queries for each Ozempic and Wegovy (Fig. 1A & 1B). Importantly, though interest in pregnancy-related GLP1 queries has exhibited noticeable growth since late 2019 (Fig. 1C), it has failed to meet the exponential rate exhibited by interest in Ozempic or Wegovy alone (Fig. 1D). The relative paucity of searches for concurrent pregnancy and GLP1 use echoes the scarcity of studies in this research area. Future research will expand to include other name-brand GLP1 drugs like ‘Mounjaro’ or generics like ‘semaglutide’ and other outcome indicators like ‘birth defects’, ‘miscarriage’, or ‘stillbirth’.